Improved Ankle Replacement Device and Method (RFT-427)
Invention Summary
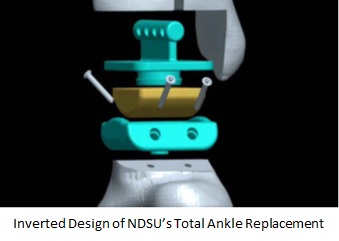
NDSU engineers have developed an improved design and material for Total Ankle Replacements (TARs) that features an inverted design, in which the concave portion of the joint is on the bottom, and the convex on the top. This inverted design and the mode of assembly and implantation offers several benefits to surgeons and patients.
Benefits
-
Maintains high surface area contact throughout range of motion, minimizing edge loading, and thereby reducing the potential for wear and fracture of components
- Range of motion similar to three component systems with stability and basic structure of two component systems performance derives from a combination of the inverted design and the snap fit of the bearing component (yellow) into the tibial component
- Inverted design maintains center of rotation and full range of motion (dorsal flexion, plantar flexion, adduction, and abduction)
- Fixation of the talar component to the bone uses screws (not solely bony ingrowth) to afford greater stabilization
- Fixation of the tibial component using an atypically shorter barrel design and a cross hole to allow bony ingrowth, enables solid anchoring with minimal tibial bone removal
- Design minimizes bone removal and stretching/navigation of the surrounding tendons resulting in a quicker surgery and shorter rehab talar dome in particular is minimally impacted to retain as much of that extremely strong bone as possible
-
Potentially reduce microparticulate wear, and thereby may lessen aseptic loosening, subsidence, and bone cyst formation
-
May be constructed with existing biocompatible implant materials
In combination, these benefits are expected to result in a much lower 10 year failure rate, and a longevity of approximately 20 years for 80% of the implants. This represents a significant improvement over current TAR designs.
An additional aspect of this invention is the use of improved materials that are expected to perform better and have even longer life expectancy than materials being used today. These composite materials are carbon fiber reinforced polyetheretherketone (CFR PEEK), which have mechanical characteristics similar to bone, have a lower wear rate, are not affected by sterilization, and are already FDA approved. This material would have a modulus of elasticity very similar to that of the surrounding bone, which we believe would significantly increase the lifespan of this TAR.
Patents
This technology is the subject of Issued U.S. Patent No. 10,568,743, and European Patent No. 29,915,89 B1, and is available for licensing/partnering opportunities.
Status
This technology is available for licensing/partnering opportunities.
Contact
NDSU Research Foundation
info(at)ndsurf(dot)org
(701)231-8173
NDSURF Tech Key
RFT, 427, RFT427
Inquire about this technology >